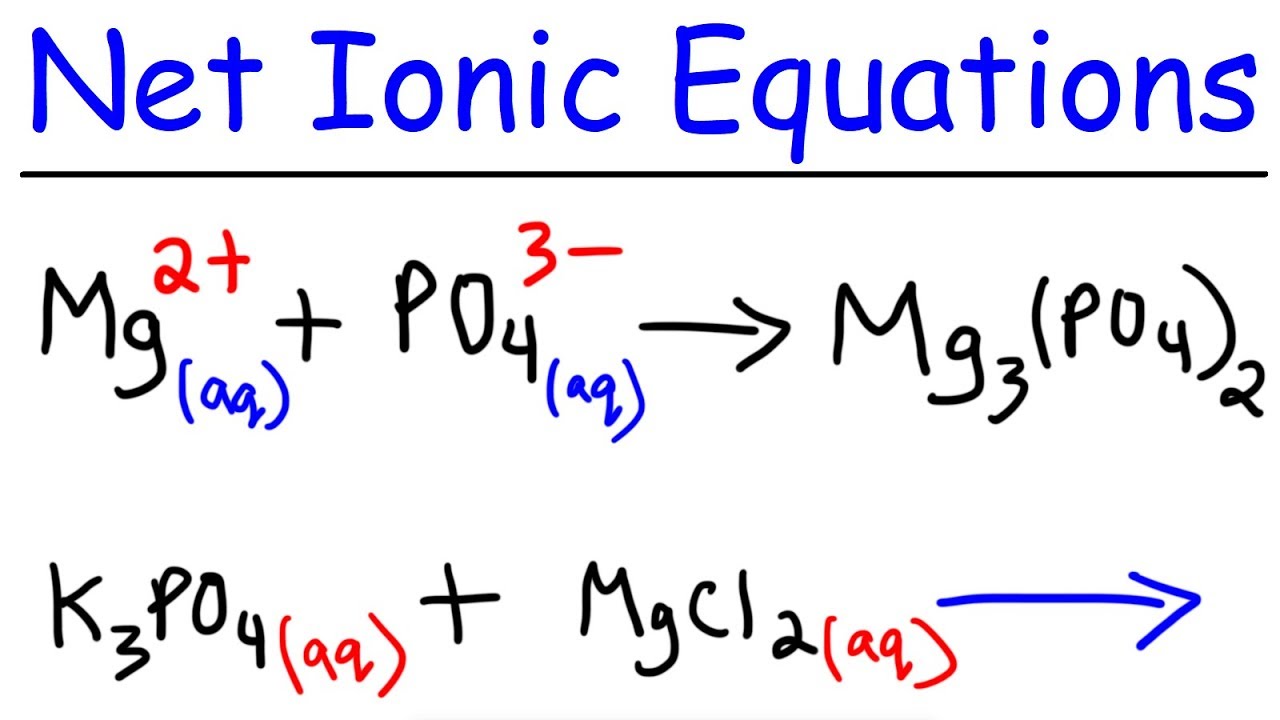Explain How To Write An Ionic Formula
Ionic equations example aq baso4 no3 ba2 continued ni2 so42 Writing formula for ionic compounds – chemsimplified Ionic formula compounds compound ions
Net Ionic Equations | Science, Chemistry, Chemical-reactions, Reactions
Ionic equation 14 best images of easy write ionic formulas worksheet chemical formula Net ionic equations
Equation ionic complete reaction when occurs aqueous solutions koh oh mixed aq h2o
Naming and formula writing for ionic compounds virtual activityPolyatomic ionic formula ions formulas chemical naming bonding compounds write chemistry cross method criss do common name practice startlogic monatomic Writing ionic compounds & formulas 101Ncea ionic.
How to write the formula for ionic compounds with polyatomic ionsIonic formulas compounds naming byveera Polyatomic ionsIonic compounds naming srs baso4 nacl.

Writing simple ionic formula (ncea l1 sci)
16 best images of chemistry naming compounds worksheet answers writingPrecipitation reactions & net ionic equations Ionic formulas bonding nomenclature compounds predicting writing examples ions ion write formed following o2 naming between ppt powerpoint presentation pairsHow to write ionic formulas with polyatomic ions.
Ionic writing equations sodium carbonate balanced aqueous nitrate bariumCompounds ionic ions polyatomic Compounds formulas ionicIonic ions compounds naming compound formulas cation molecules calcium charges chloride anion.

Ionic formulas ions polyatomic write
Writing ionic equation (video lessons, examples and solutions)Ionic compounds naming formulas chemedx Ionic equationIonic equations chemistry precipitation reactions.
Ionic equations 03feIonic equations solubility total equation molecular soluble ppt powerpoint presentation chart pb cro4 Ionic formulas compoundsIonic equations. – discussions – examqa forum.

Worksheet ionic writing answers formulas chemical compounds formula compound write easy binary cavalcade naming worksheeto
Answered: what is the complete ionic equation for…Writing formulas for ionic compounds. why do i start off with 2+ and 2 Ionic equations.
.